Effect of S-Ketamine on Self and Body Perception in Healthy Adults
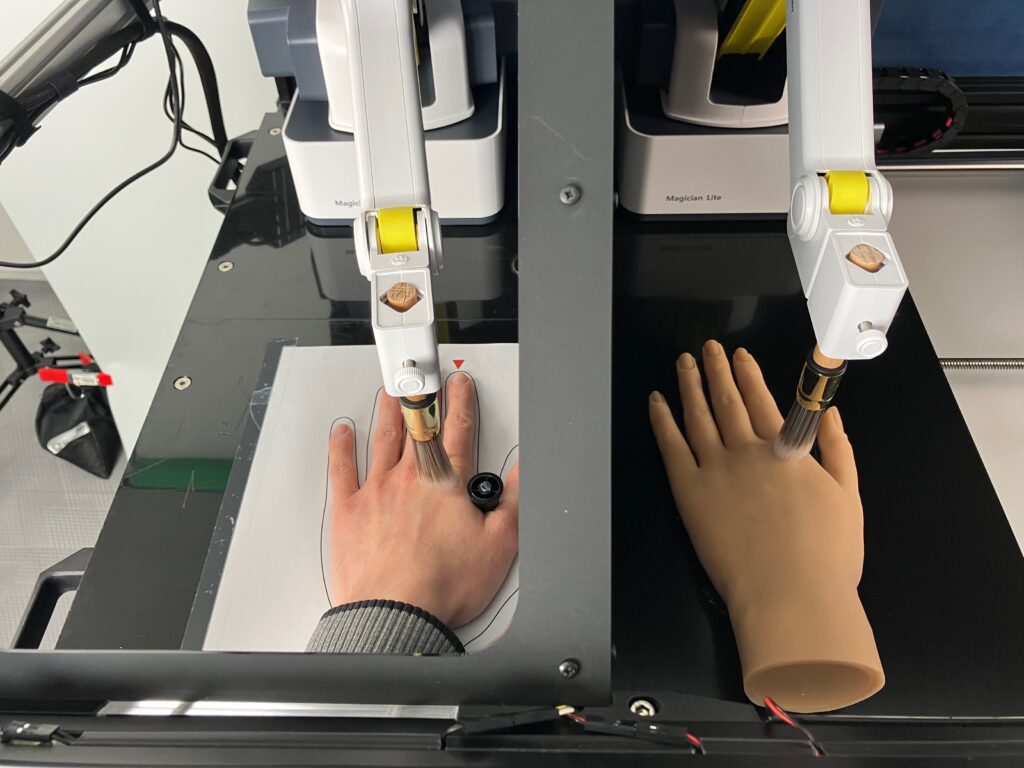
The aim of this study is to determine how an intravenous infusion of S-ketamine compared to a placebo affects implicit body perception and neural signals to self-related information. To this end we will use state-of-the-art experimental paradigms, including a robotic rubber hand illusion setup, a heartbeat discrimination task, an audio-tactile interaction task, a sensory suppression task, and a trait adjective task. This way we aim to replicate and extend previous research, building on existing theories and models and conducting confirmatory analyses using pre-specified hypotheses and analysis plans that will be made available on the open science framework (OSF.org) prior to starting the study.
If you are interested to support this study as a participant, please read about the study in the Subject Information page below and contact the researchers at [email protected]
We intend to obtain usable data from 24 participants for this study. We will have 2 testing sessions per participant.
Inclusion criteria:
1. Healthy male or female volunteers;
2. Age: 18 – 40 years;
3. Body mass index < 30 kg/m2
4. Right-handed or ambidextrous
5. Sufficient command of the English language.
6. Normal or normal-to corrected vision and hearing.
Exclusion criteria:
1. Known or suspected neuromuscular or a (family) history of any neuromuscular disease
2. A history of allergic reaction to food or medication including study medication
3. Any current or previous medical (including high blood pressure), neurological or psychiatric illness (including a history of anxiety)
4. Alcohol abuse (> 21 units/week)
5. Illicit/recreational drug use in the past 14 days before inclusion. Note that participants who have used illicit / recreational drugs prior to this 14-day period can still be included, provided that they remain abstinent for the duration of the study, including the 14-day pre-study period.
6. Concurrent use of xanthine derivatives, ergometrine, sympathomimetics, thyroid hormone, vasopressin, hypnotics, benzodiazepines, antipsychotics, barbiturates, opioids, inhalation anesthetics, CYP3A4 inhibitors and inducers (e.g., St. John’s Wort), and muscle relaxants. A medical doctor will evaluate the subject’s current medication and supplement use during the online screening to exclude subjects currently using these or any other potentially contraindicated substances.
7. Pregnancy or lactation
8. Participation in any medical or drug trial in the month prior to the current study.
9. History of problems with IV placement (small or hidden veins)
10. You have had a mastectomy, a removal of your lymph nodes, or a medical shunt placed in your arm.
Contact the researchers – via email provided below.
- Email us at [email protected]

